Pool Shock & Sanitation
It is essential to use pool shock and sanitizers to prevent the growth of fungi and bacteria and to maintain the health of swimmers. In the following content, you will find information and the best prices on all types of pool shock and sanitizers.
Pool Shock & Sanitation Expert Buying Guide and Price List
When the chemical properties of the water are within the standard range and in a favorable and balanced state, the pool water should be very clear and free of any unpleasant odor.
This factor preserves the health of the water and swimmers and, at the same time, prevents problems in the operation of the pool's mechanical and damage to the pool's structure.
In fact, water is a solvent that more or less dissolves mineral substances; water that is chemically balanced is neither corrosive (acidic) nor produces sediment (alkaline).
On the other hand, the chemical imbalance of water causes the growth of microorganisms (algae, fungi, and bacteria), changes the smell and color of water, increases corrosive properties, and damages the structure and equipment of the pool.
Aluminum sulfate is a common coagulant substance that is injected into the pool water before the filter to coagulate the suspended substances in the water and make them into larger particles that can be easily absorbed and separated by the filter.
When the coagulant is added to water, an insoluble jelly-like sediment called floc is formed in the water due to the reaction of alkaline substances.
The floc absorbs colored compounds and organic matter and traps them so the filtration system can separate these flocs from the water, and as a result, clean water comes out of the pool filter.
Note: Aluminum sulfate must be added on a schedule, otherwise, the floc may form inside the pool and make the water cloudy. The floc should be added into the pool water suction tank in a way that there is enough time for the formation of a floc.
Types of Pool Shock & Sanitation Equipment
The types of materials used to sanitize and disinfect swimming pools are as follows:
Chlorine
Chlorine in any form (powder, tablet, liquid, or gas), when added to water, reacts with hydrogen and oxygen molecules in water and forms hypochlorous acid and hydrochloric acid; these two substances have very good disinfection properties to Eliminate harmful bacteria and microorganisms.
Chlorine has a direct relation with the pH of water, and the best condition for absorbing chlorine in water is when the pH value is between 7.2 and 7.4.
If the swimming pool is in the open air, the continuous sunlight causes the quick waste of the remaining chlorine. It requires the use of a chlorination device with a larger capacity.
Note: When charging chlorine, the chlorinator must be installed in the inlet and outlet of the chlorine valve to prevent water overflow. When you close the inlet valve of the chlorinator, the water level drops, and at that time, the device can be charged, which does not require aeration.

Ozone (O3)
This material is one of the most expensive materials for disinfecting swimming pools, but its side effects, such as eye irritation and respiratory problems, are far less than chlorine.
Ozone is not usually used on its own, and chlorine is often used as a supplementary disinfectant along with ozone. The combination of these two together reduces chlorine consumption and improves the disinfection quality of swimming pools.
Note: In order to prevent pool water from entering the ozone generator, it is better to install the ozone injection device in such a way that the height of the device is higher than the water level of the pool and jacuzzi, or you can use a one-way valve in the venturi part of the ozone injection device.

Ultraviolet (UV)
Using an ultraviolet lamp AKA UV lamp is another method of disinfecting pool water. In this method, the pool water is exposed to ultraviolet rays and disinfected by passing through the closed chamber of the lamp.
The use of the UV lamp depends on the circulation of the pool water so that the disinfection operation is carried out well. By using this system alone, it is not possible to directly prevent the growth of algae in the pool bowl.
Therefore, using a UV lamp alone is not a good way to disinfect pool water, but some disinfectants, such as chlorine, should be used along with it. Of course, ultraviolet rays also destroy pathogens that are resistant to chlorine, such as Legionella pneumophila and Pseudomonas aeruginosa.
It should be noted that UV rays do not change the physical and chemical properties of water. Therefore, it does not contaminate the water with chemicals and keeps the coordinates of the water naturally; the pH of the water does not affect the disinfection.
UV is used in the pH range of 6-8.5. The UV device is installed directly in the path of the water pipe, which does not require additional plumbing and space.
The service and maintenance of the device are simple; the water pressure drop inside the device is negligible, and the device does not have mechanical depreciation.
 |
 |
Review, Selection, and Pricing of HVAC Equipment
Chemical Attributes of Water
The most important chemical factors of water are as follows:
Water pH (Standard Range 7.6 to 7.4)
A low pH value is a sign of the acidity of the pool water, which causes swelling of the skin and eyes of swimmers in addition to corrosion in the pool structure and equipment. In this case, chlorine is absorbed by water faster and loses its antiseptic properties.
On the other hand, a high pH value means that the water is alkaline, which will cause the pool water to become cloudy and create deposits on the pool floor and walls, as well as the pipes and facilities of the pool (adding sodium carbonate increases the pH value and adding hydro Chloric acid (dry acid) will reduce the pH value in the pool water.
The Alkalinity of Water (Standard Range 80-150 ppm)
One of the parameters that are considered in measuring the chemical properties of water in pools is the alkalinity of the water.
Setting this factor in an acceptable range will prevent strong fluctuations and sudden changes in the pH value of the water. In any case, the appropriate amount of alkalinity in water should be between 80 and 150 ppm.
If the concentration of these substances is less than 80 ppm, even with the high pH of the water, the amount of acid added is more than usual, and if it is more than 200 ppm, it will be very difficult to adjust the pH in a normal range.
As a result, before adjusting the pH value, it is necessary to measure the concentration of alkaline substances in the water.
Water Hardness (Standard Range 200-400 PPM)
Water hardness or calcium is a measure of the amount of one of the alkaline substances (calcium) in the water, the excess amount of which is removed from the solution state and deposited on the wall, floor, and inside the water circulation devices of the pool.
Water hardness is the most important factor in the formation of sediment in swimming pools, and the best way to deal with it is to use resin hardener with cationic resin.
Also, note that if the water hardness is less than the permissible limit, it will damage the covers, colors, and concrete shell of the pool bowl.
TDS (Suspended Particles "Less Than 1000 PPM" in Water)
TDS is a measure of all suspended particles and solid components in pool water that have not been separated by passing through the filter and mainly includes solid compounds of chlorine, fats, and pollution.
It should be noted that the concentration of these particles should not reach 1000 ppm. The easiest way to reduce the TDS value of pool water is to replace part of the pool water with fresh water (siphoning pool water).
Determining Resin Softener Capacity
Water softener capacity is the amount of hardness that a water softener can remove from water before it needs to be regenerated. Water hardness is measured in grains per gallon (GPG). One grain per gallon is equivalent to 17.1 milligrams per liter (mg/L) of calcium carbonate (CaCO3).
Water softener capacity is an important factor to consider when choosing a water softener for your home. It refers to the amount of hard mineral content a water softener can remove before it needs to enter its regeneration cycle.
Capacity is measured by the amount of hardness it can remove before it regenerates, while the level of hardness in water is measured by “grains per gallon” (GPG) or parts per million (PPM).
To correctly size a water softener, you’ll need to know your water hardness and your average daily water usage. You can find out your water hardness by using a testing kit or contacting your local water supplier.
You can estimate your water usage by multiplying the number of people in your household by the average gallons per person per day (usually around 75 gallons).
The following formula will help you to determine the hardening capacity:
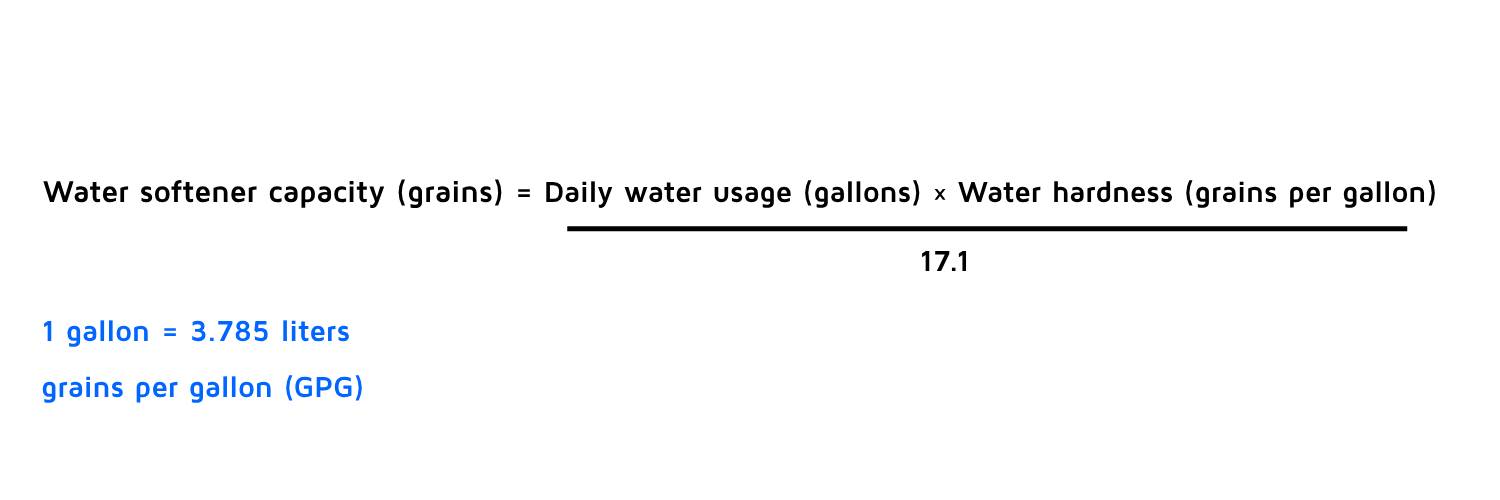 |
 |
Buying Pool Shock & Sanitation Supplies
To choose and buy suitable pool water disinfection equipment or any other pool supplies, we must see what kind of disinfectants are suitable for our desired location.
Pool water disinfection equipment has 3 types of chlorine, ozone (O3), and ultraviolet rays (UV) disinfectants, each of which has a different efficiency for disinfecting and disinfecting pool and hot tub water.
On this website, the technical information of dozens of models of pool and hot tub water disinfection equipment from different brands with original warranty is provided, so that you can enjoy the good feeling of optimal and smart shopping.
The Price of Pool Shock & Sanitation Supplies
The price of pool water disinfection equipment is based on the capacity and volume of the pool, the type and model of the device, the amount of water, the power of the lamp (for the UV lamp device), the amount of disinfectant liquid weight, etc., according to your needs and budget, choose the best model from dozens of models of pool water disinfection equipment on the site of DamaTajhiz HVAC.
It should be noted that the prices of the swimming pool shock and sanitizing equipment provided on DamaTajhiz HVAC website are all up-to-date, and the discounts desired by the consumers have already been included.
Dear Users:
However, when choosing a product brand, history, brand strength, service, and product price are key factors.
But in the field of pool deck drains and gratings, most of the users and buyers select the top-selling brands like DamaTajhiz, Emaux, Hayward, Hyperpool, Aquamarine, and so on, based on quality, price, and service.
DamaTajhiz HVAC provides all types of pool shock and sanitation products in the Middle East region. If you need a pool and deck drain for any of the countries in the region, you can place your purchase order with our sales experts.
"Knowledge Fuels Better Choices"
Registered Trademark and Stewardship Business Licenses Issued by the Union of Virtual Business Association and the HVAC Equipment Industry.
DamaTajhiz HVAC Participation at International HVAC and Construction Facilities Exhibitions Demonstrates its Global Reach and Commitment to the Industry.
We Look Forward to Your Call and the Opportunity to Meet With You
SHARE THIS CONTENT TO SPREAD THE KNOWLEDGE
| |
Head Office: No. 463,Talebian Alley,Taleghani St.Tehran,Iran


DamaTajhiz has provided the opportunity to sell and ship specialized HVAC equipment for applicants in the following countries as the first and the most popular online store for selling HVAC equipment (Heating , Ventilation , Cooling , Air conditioning) in the Middle East : Afghanistan – Tajikistan - Uzbekistan – Turkmenistan – Azerbaijan – Armenia – Georgia – Turkey – Iraq – Syria – Jordan – Kuwait – Emirates – Qatar – Oman.

















